👑慧智非侵產前染色體篩檢v1.0/ v2.0/ v3.0 (NIPS)
服務介紹
■ 高檢出率 零流產 產前篩檢
依據衛福部統計,全台每年約有4萬多名媽媽選擇羊膜穿刺,但平均有40~120多個寶寶產生不必要的併發症,甚至媽媽因感染而敗血死亡的案例。
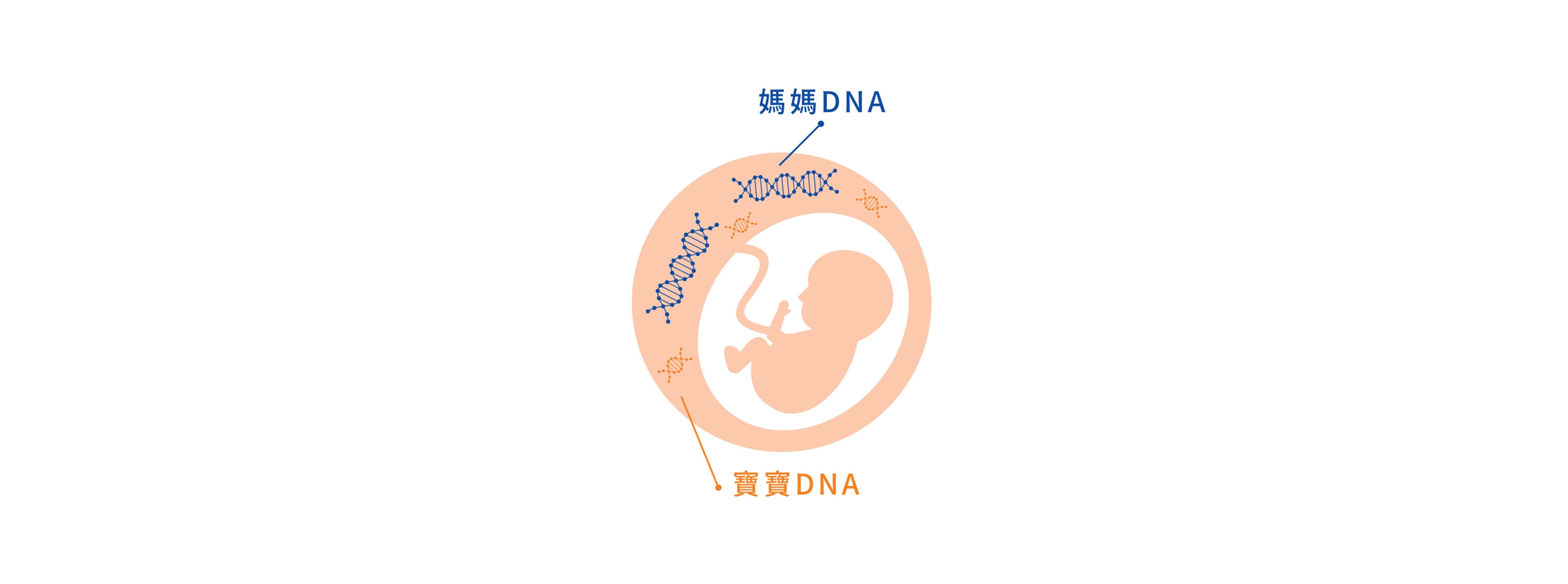
"非侵產前染色體篩檢NIPS"透過抽血的採檢方式結合次世代定序(NGS),將媽媽血液中胎兒游離DNA進行定序,精準且快速確認寶寶染色體/基因是否異常。
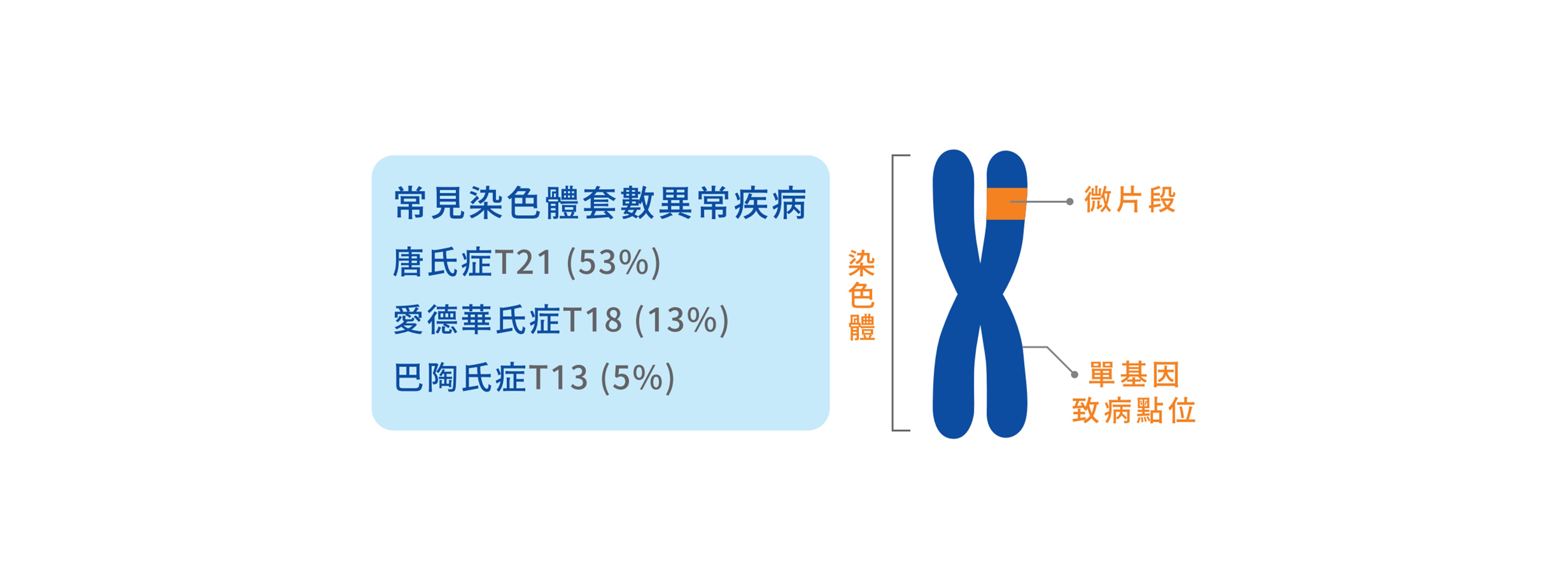
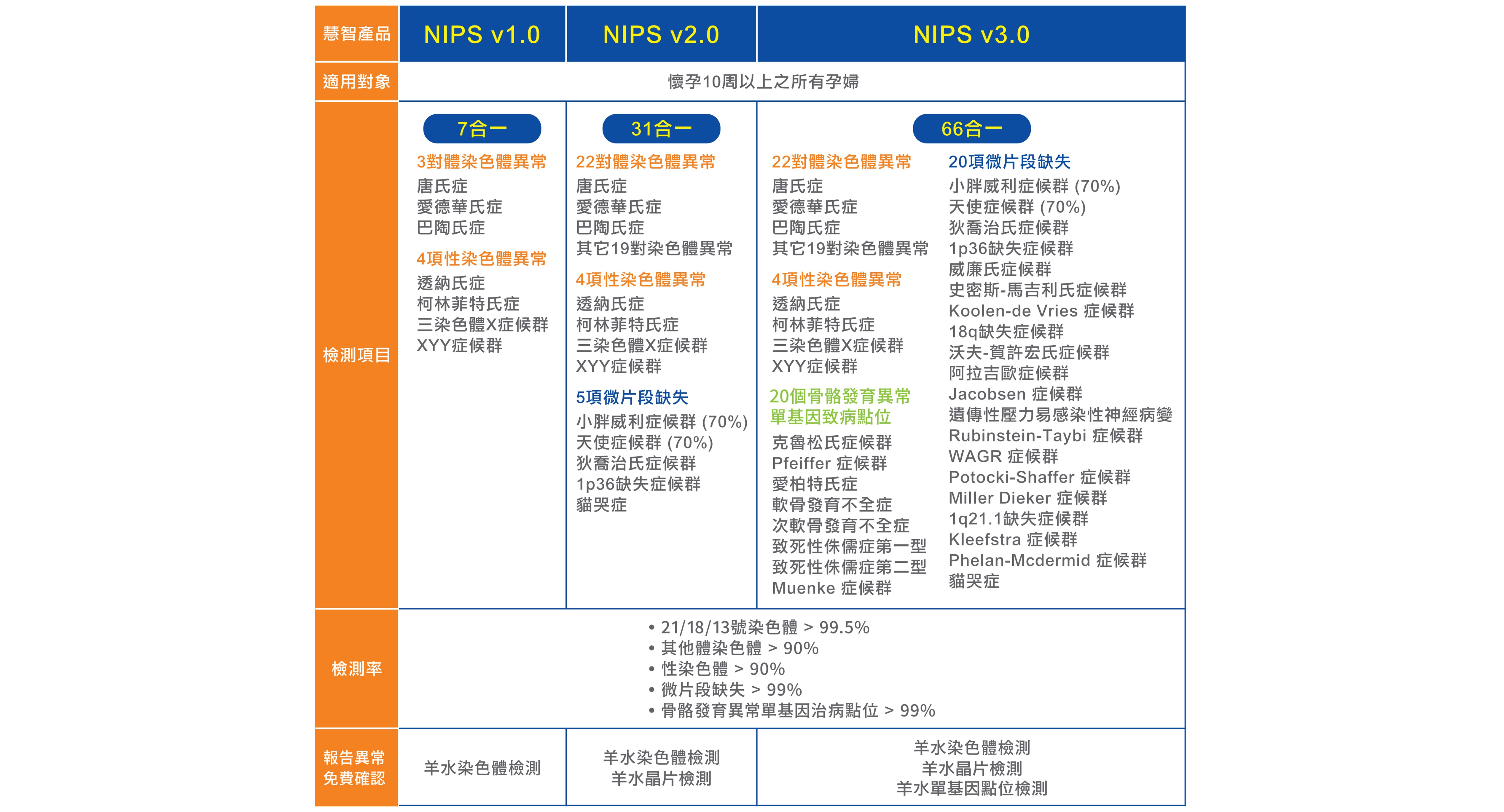
除了染色體檢測外,微片段和單基因致病點位檢測是超音波檢查較不容易發現的。 據臨床統計,這些基因異常容易造成發育遲緩、 器官發育不全及智能障礙等嚴重疾病。
→ NIPS加驗微片段及單基因致病點位,提早給寶寶完整檢查。
■ 慧智非侵產前染色體篩檢 保障多,更安心
檢測說明
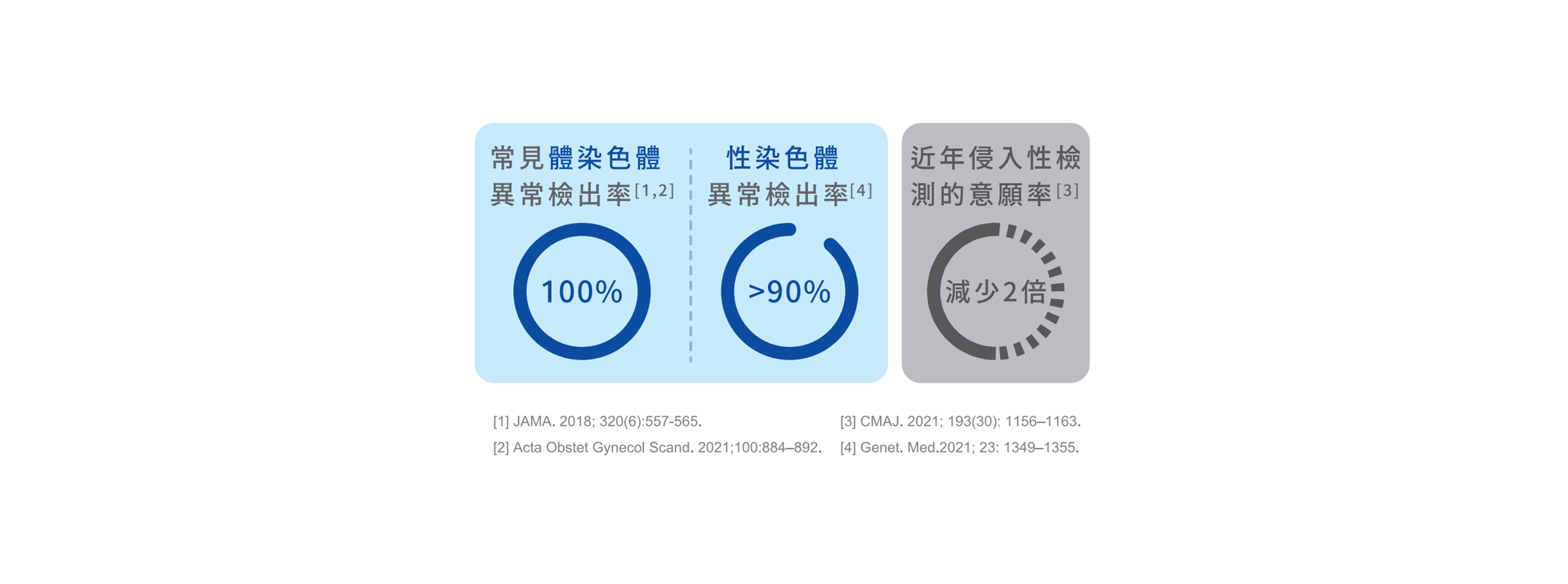
■ 國際臨床試驗數據
近幾年各國醫療院所陸續發表產前篩檢NIPS的相關研究,結果顯示:
適用對象
目前多個學會推薦非侵入性檢測,並且建議孕婦在完整瞭解檢測方法、風險、優缺點及確診方法等資訊後,選擇檢測。每位懷孕超過10週且有以下考量的孕婦都可以考慮NIPS:
✔ 害怕侵入性檢查的孕婦
✔ 超過34歲之高齡孕婦
✔ 超音波檢查發現異常,懷疑胎兒為染色體異常的孕婦
✔ 有先天性異常的家族史或者曾生過先天性異常寶寶者
✔ 唐氏症血清生化篩檢高風險的孕婦
✔ 人工生殖、卵子捐贈或懷有雙胞胎的孕婦
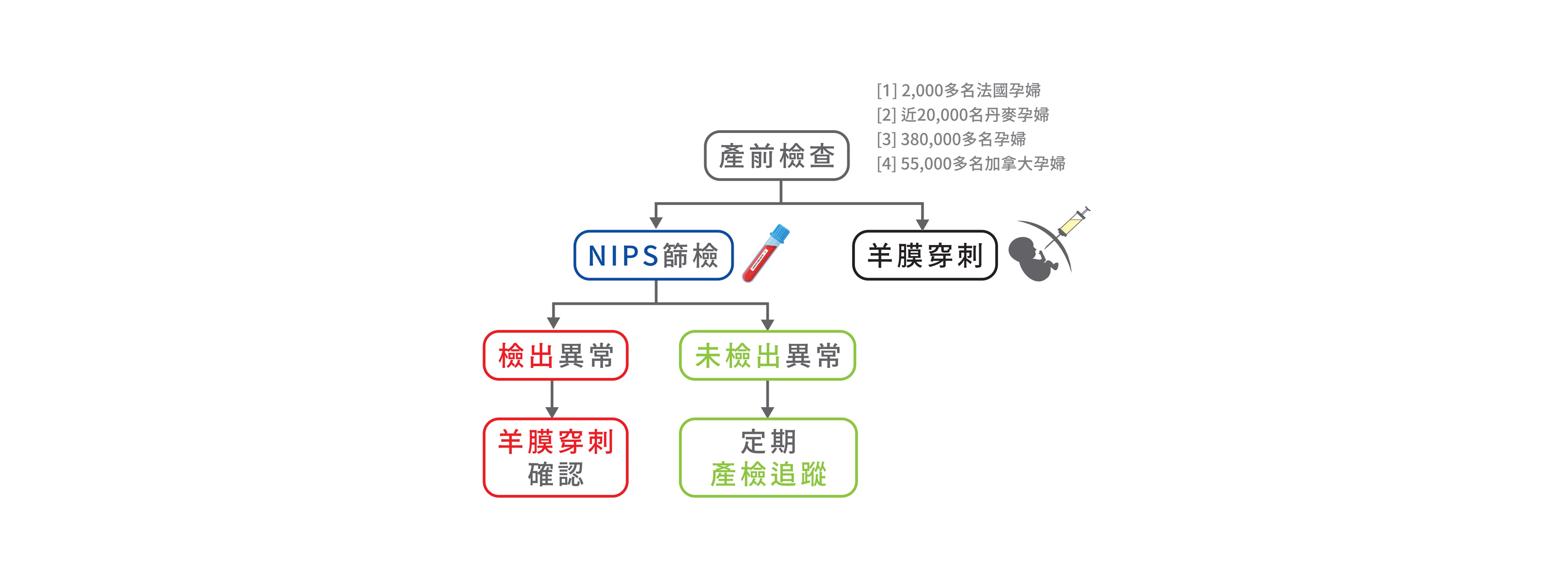
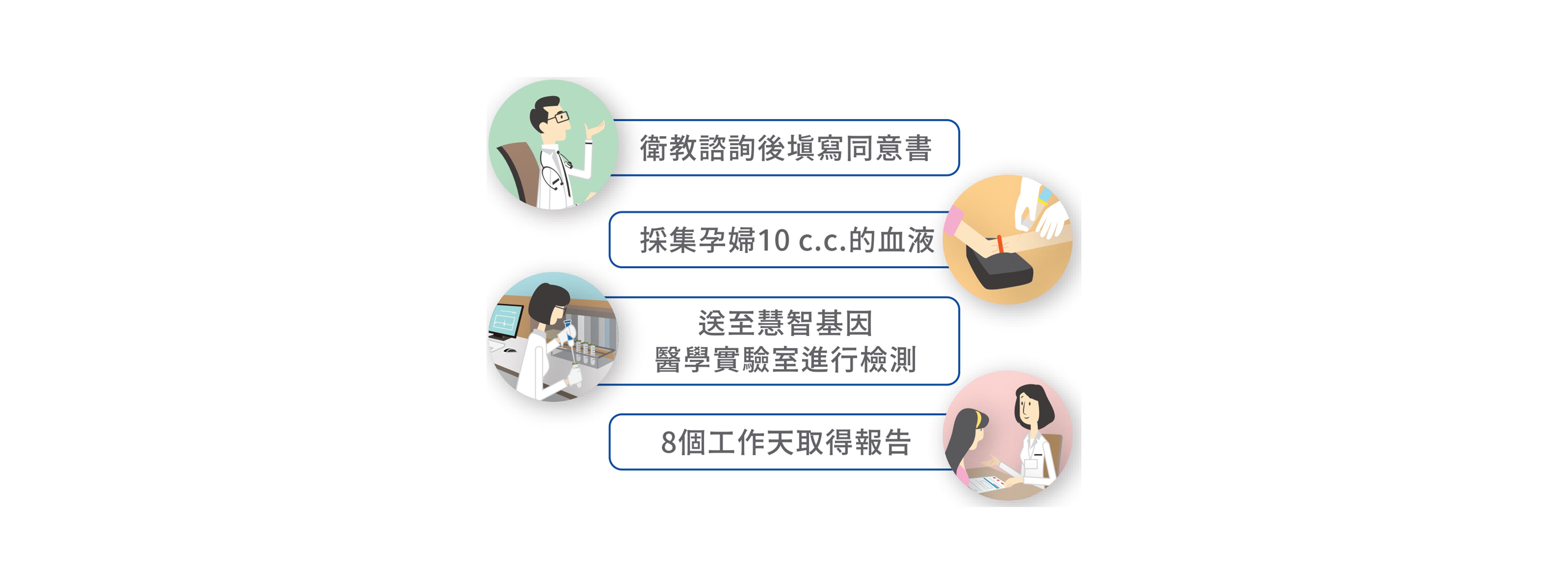
檢測流程
其他說明
Q:我不是高齡媽媽也需要做嗎?
A:雖然高齡產婦懷有異常寶寶機率較高,但國際學會統計,微片段及單基因異常發生機率與媽媽年齡無正相關,大多為自體突變,所以建議懷孕婦女不論年紀及胎次,每一胎都應進行產前篩檢。Q:收到檢測報告,我應該做甚麼?
A:• 報告顯示檢出異常,建議進行免費羊水染色體或晶片檢測,以確認篩檢結果。→建議接受進一步遺傳諮詢或與專科醫師討論
• 報告顯示未檢出異常,仍需定期產檢追蹤寶寶的情況,由產檢醫師綜合評估完整孕期。
Q:會對寶寶造成甚麼影響?
A:完全不會!因為NIPS只需採集血液,不需使用侵入性羊膜穿刺就可以取得寶寶的DNA,是一種零流產的安全篩檢方式。
慧智優勢