子宮內膜癌基因分型
服務介紹
子宮內膜癌是110年台灣女性癌症十大發生率中的第五名,每年近3000位女性罹患該癌症,依不同的病理組織形態又可分成不同的組織分型,其中最常見的為子宮內膜樣癌 (Endometrioid carcinoma),佔了發生率的80%以上,其次為預後較惡性的漿細胞癌 (Serous carcinoma)。
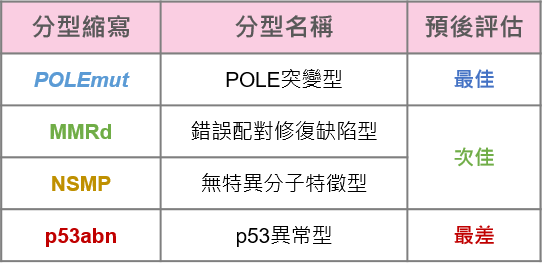
而根據基因表現,子宮內膜樣癌的惡性程度也不相同,為了讓病患接受更適切的治療方式,FIGO、NCCN等國際醫療指引中,皆建議在初步評估子宮內膜癌時,就應該加入基因分型的方式,將患者分為四個子型,分別為:
🔸POLE突變型 (POLE mutation, POLEmut)
🔸錯誤配對修復缺陷型 (mismatch repair deficiency, dMMR)
🔸無特異分子特徵型 (no specific molecular profile, NSMP)
🔸p53異常型 (p53 abnormal, p53abn)
■ 基因分型與癌症治療
對於子宮內膜癌的治療方式多為手術切除後,再依照病患癌症的風險高低給予輔助性治療。而透過慧智子宮內膜癌基因分型加上現行臨床評估的方式,可以更有效、更全面性的評估病患腫瘤的惡性程度,找出預後最好及最差的患者,協助醫生分別給予最適切的治療方式。
檢測說明
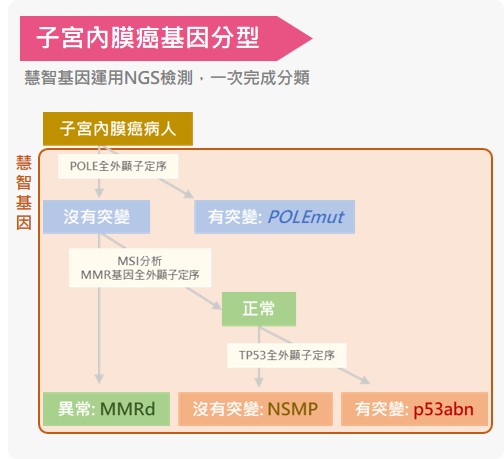
該檢測包含POLE、MLH1、MSH2、MSH6、PMS2、TP53的全外顯子分析,並額外進行微衛星不穩定性MSI分析,符合國際指引的分型方式,完整評估基因分型。
適用對象
子宮內膜癌病患:
◆ 病患初診斷為子宮內膜癌時。
◆ 病患接受手術治療後。
◆ 病患欲接受免疫治療前。
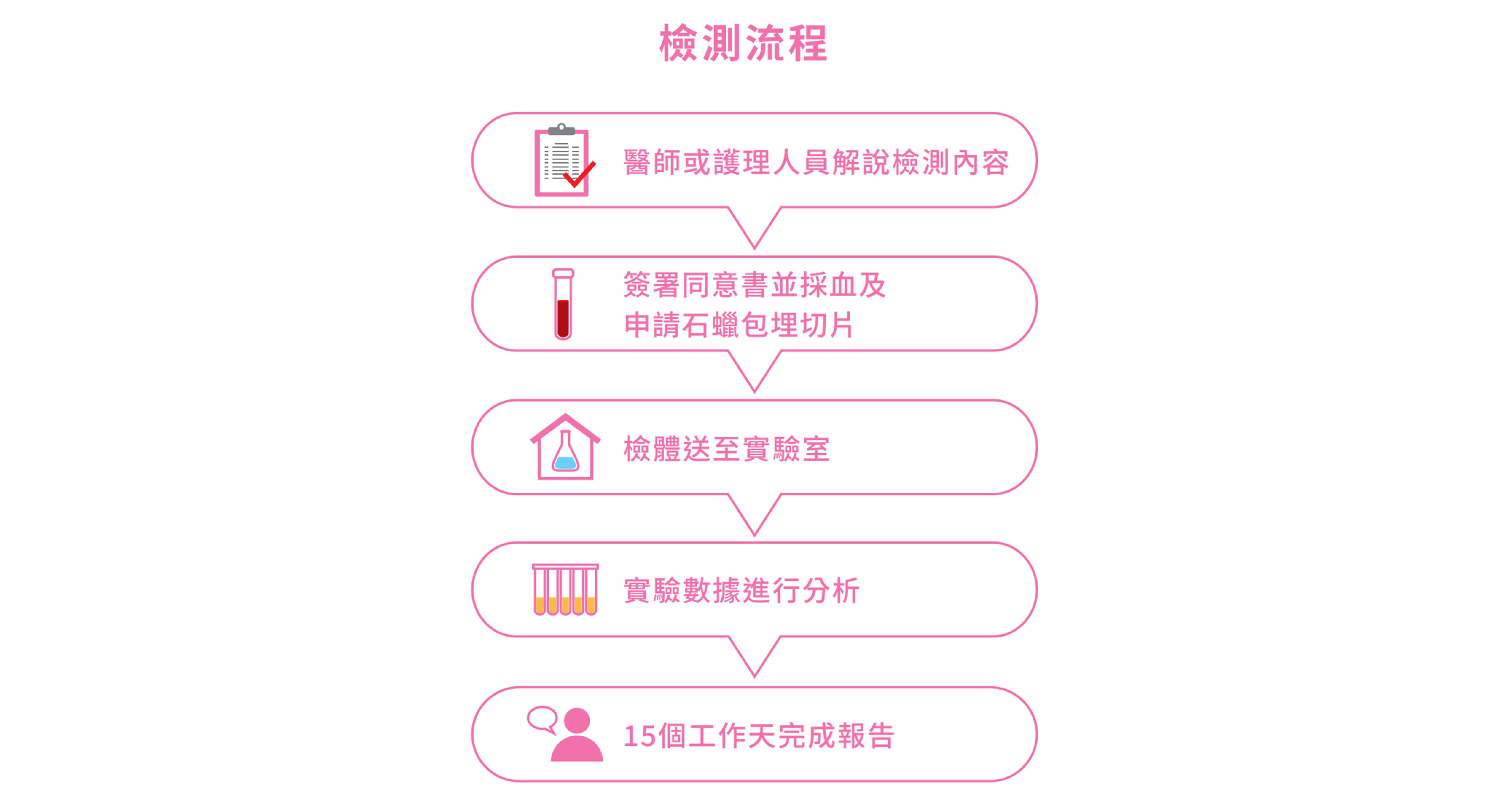
檢測流程
慧智優勢

