👑慧智CGP癌症基因檢測
廣泛分析癌症基因,提供MSI、TMB、LOH資訊
服務介紹
■ 為什麼要做慧智CGP癌症基因檢測?
隨著醫療科技的進步,癌症精準治療的概念已逐步成熟,透過基因檢測了解癌症的突變,就可以針對病人的癌症對症下藥。以肺癌為例,2004年科學家對於肺癌基因突變的了解較少,而2020年已經發現肺癌有數十種不同的驅動基因(Driver gene),其中部分基因突變已經有標靶藥物可以進行治療。隨著醫學對於癌症突變更加透徹,檢測技術也須不斷更新,提供臨床完整、全面的基因分析。

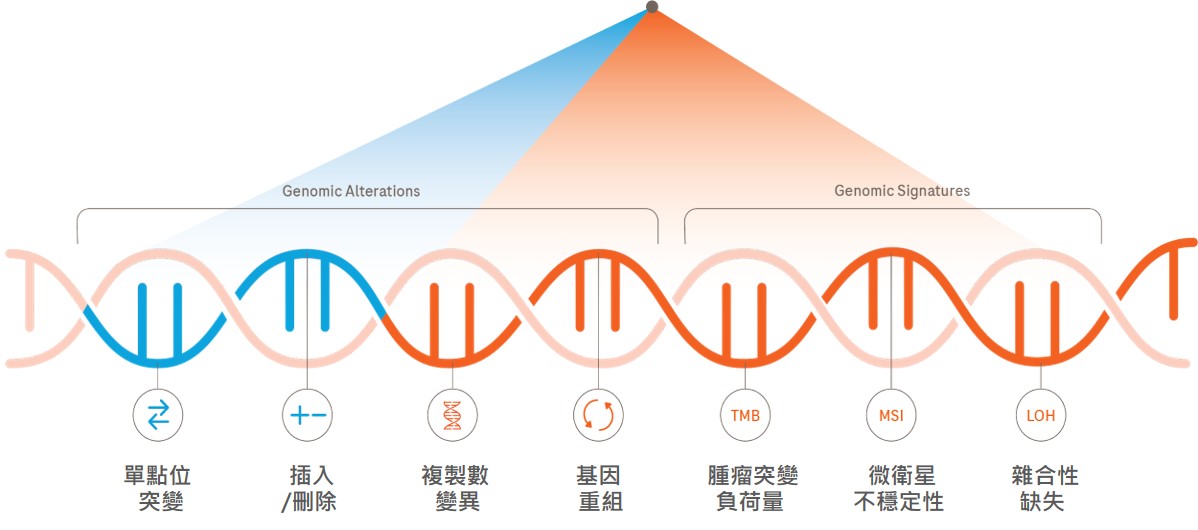
慧智CGP基因檢測使用與FoundationOne®相同的檢測技術與分析平台,並且全程在台完成檢測,提供更迅速且便利的檢測服務。 檢測內容與FoundationOne®相同,包含:
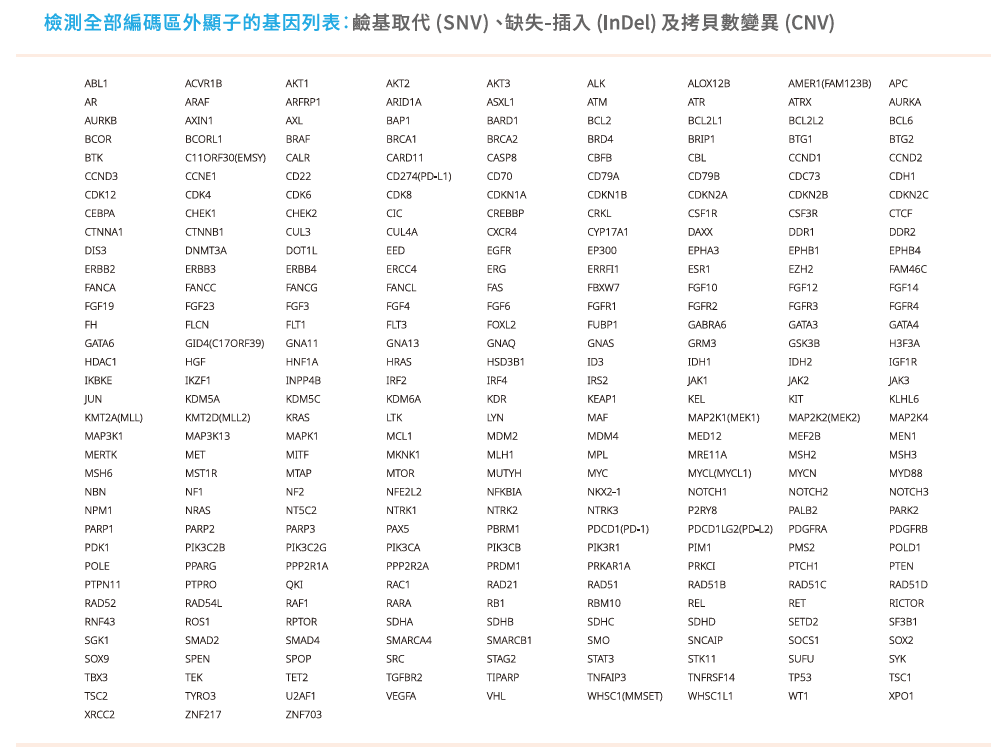
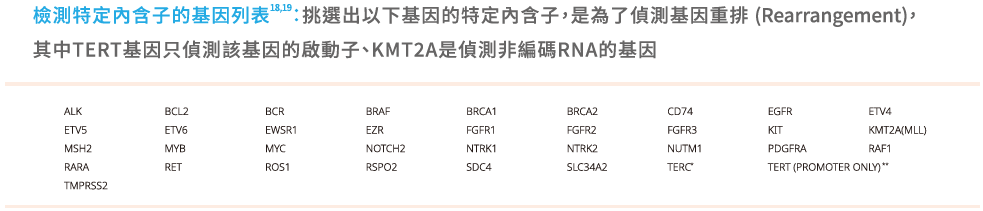
- 相同的檢測範圍: 檢測檢測324個基因,偵測SNV、indel、CNV及Fusion,以及三種基因特性MSI、TMB、LOH。
- 相同的檢測技術: 使用Hybrid capture-based NGS定序,以連續性的探針偵測目標基因,擁有更佳的樣品偵測複雜度 (Complexity) 及基因覆蓋均勻度 (Coverage uniformity)。
- 相同的生物資訊分析平台: 使用FoundationOne Analysis Software加上AVENIO Connect Software進行分析,此分析平台經過500,000個以上臨床樣品證實,包含100種以上的癌別,並且即時更新資料庫。
檢測說明
■ 慧智CGP癌症基因檢測: 廣泛分析癌症基因
慧智基因共分析324個基因,並提供三種基因特性(MSI、TMB、LOH)。
適用對象
◆ 所有實體癌症病患
◆ 欲評估標靶治療,或申請健保藥物給付時
◆ 欲找尋標靶治療相關臨床試驗之病患
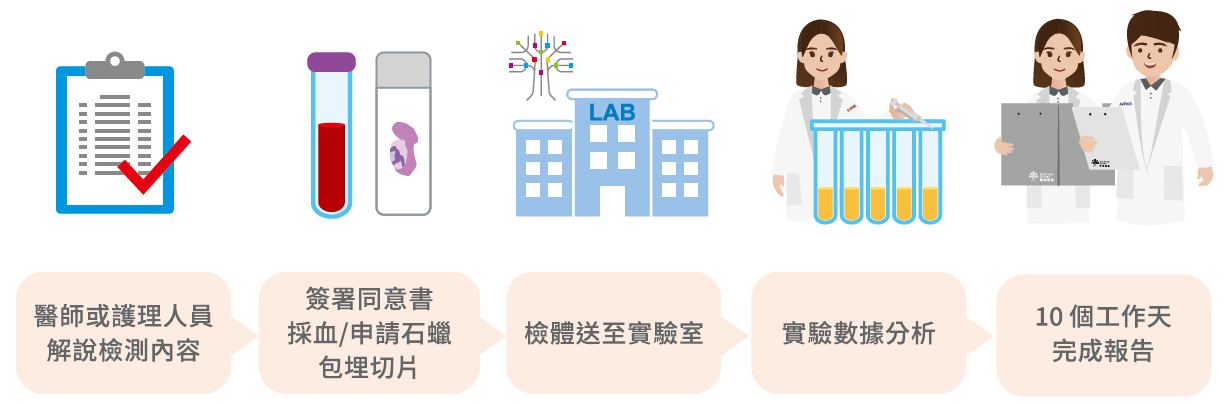
檢測流程
慧智優勢

