👑慧智HRD檢測
找出適用PARP抑制劑治療的病患
服務介紹
■ 什麼是慧智HRD檢測?
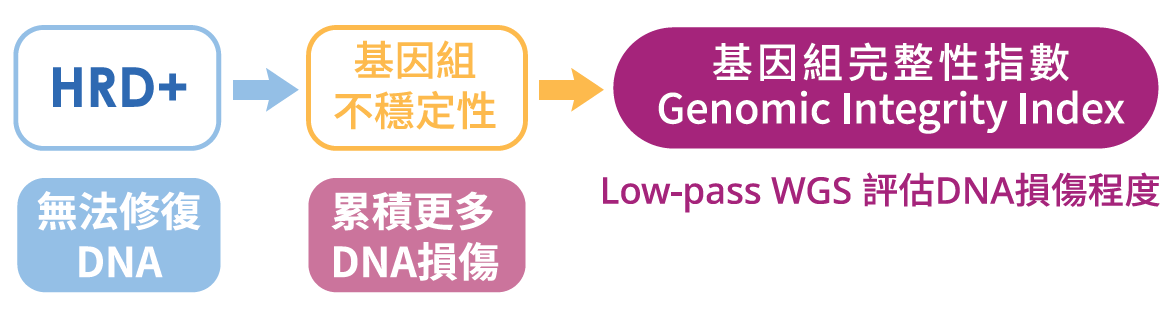
慧智HRD檢測是一種可以協助醫師評估癌症病患是否適合接受PARP抑制劑治療的基因檢測。利用NGS次世代分析技術,檢測內容為:
1. 包含BRCA1/2在內的28個HRR基因
2. 基因組完整性指數 (Genomic Integrity Index, GII)
HRR基因異常或是GII異常的病患,可與醫師討論是否適用PARP抑制劑 (PARPi) 進行治療。
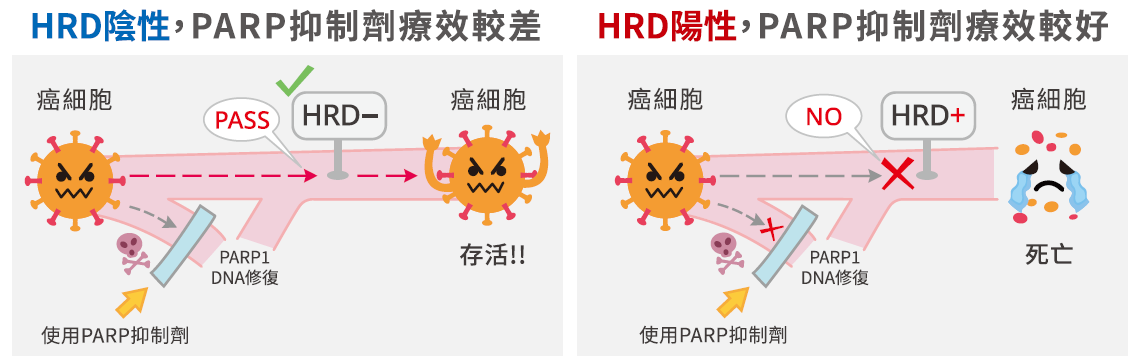
HRR是一種雙股DNA斷裂修復機制,當細胞中的DNA受到損傷時,透過HRR可將DNA修復為正常。慧智HRD檢測可以分析癌細胞的HRR功能,若檢測結果為陽性,代表癌細胞可能發生HRR修復機制異常,只剩下單股斷裂的修復機制。對於HRD陽性患者來說,目前最新的治療方式為口服標靶藥物 -- PARP 抑制劑,其透過干擾癌細胞內單股斷裂的修復機制使癌細胞死亡。
檢測說明
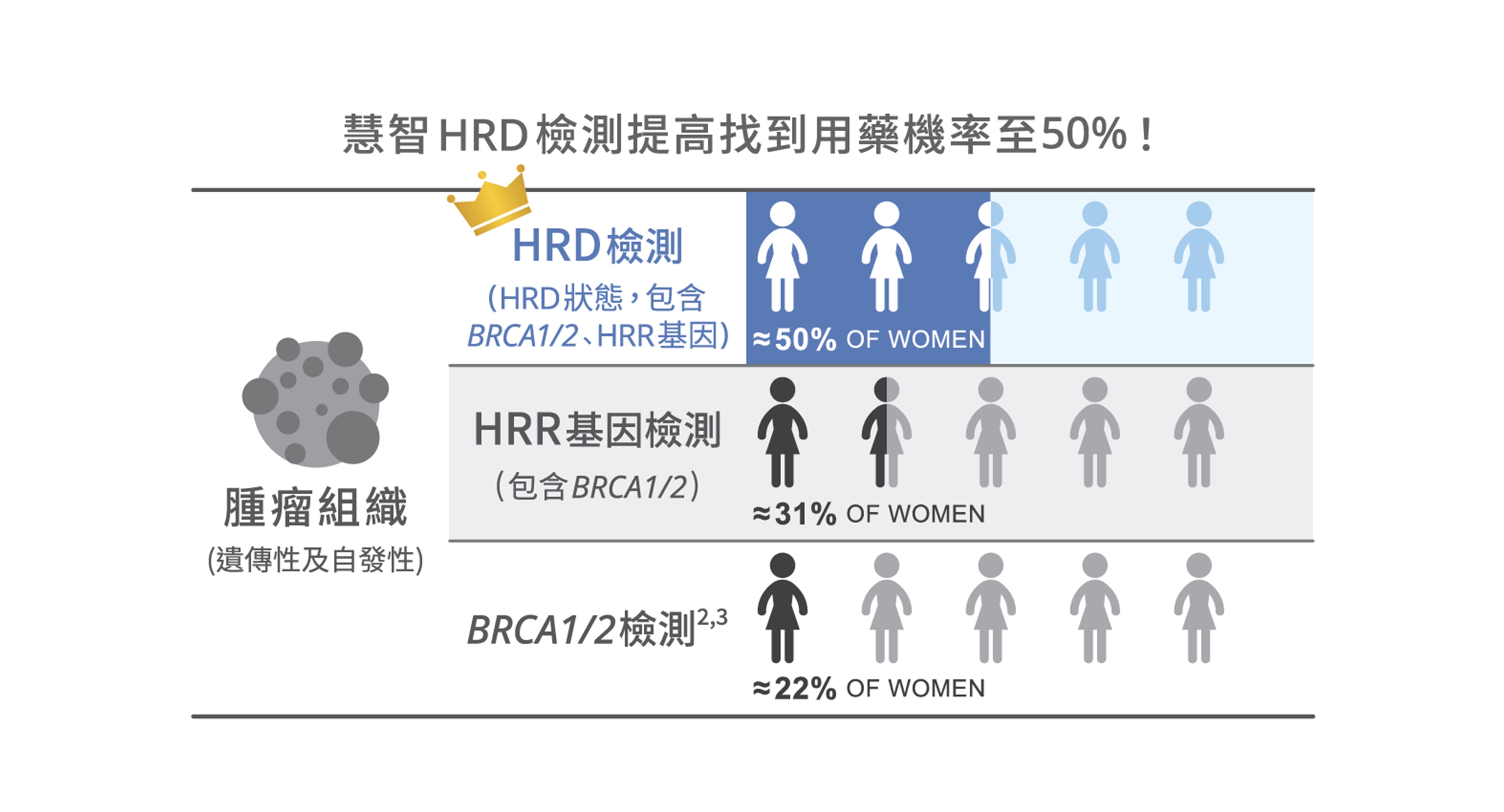
■ 慧智HRD檢測可以找到更多適合用藥的病人
以卵巢癌為例,若僅檢測BRCA1/2基因突變,約只能找出22%的適合PARP抑制劑的病患,而慧智HRD檢測可以找出約50%適合用藥的病患,提升病患用藥的機會。
適用對象
◆ 欲使用PARP抑制劑治療之患者(例如卵巢癌、攝護腺癌、乳癌等)
◆ 檢測過BRCA1/2,未發現突變,但仍希望使用PARP抑制劑治療之患者
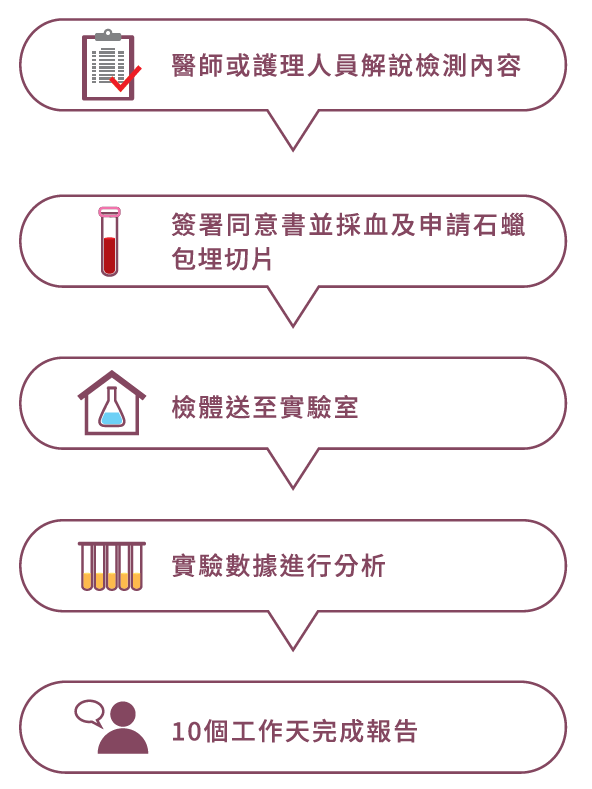
檢測流程
慧智優勢

