👑慧智癌監控基因檢測 v1.0/ v2.1/ v2.2/ v3.0
服務介紹
■ 基因突變與標靶治療
隨著醫療科技的進步,癌症精準治療的概念已逐步成熟,透過基因檢測了解癌症的突變,就可以針對病人的癌症對症下藥。
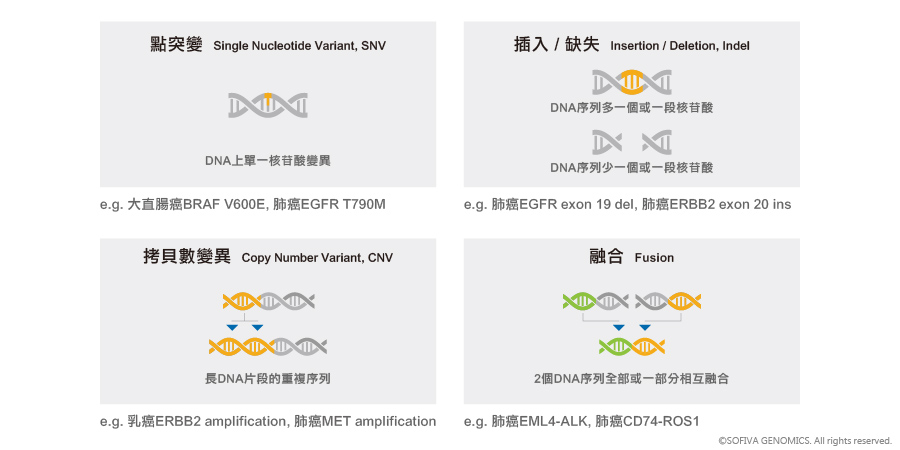
慧智癌監控運用次世代基因定序(Next Generation Sequencing, NGS)技術,透過一次檢測了解不同癌症的基因突變,包含點突變(SNV)、插入/缺失(indel)、拷貝數變異(CNV)及融合(Fusion),提供病患精準又有效率的用藥資訊。
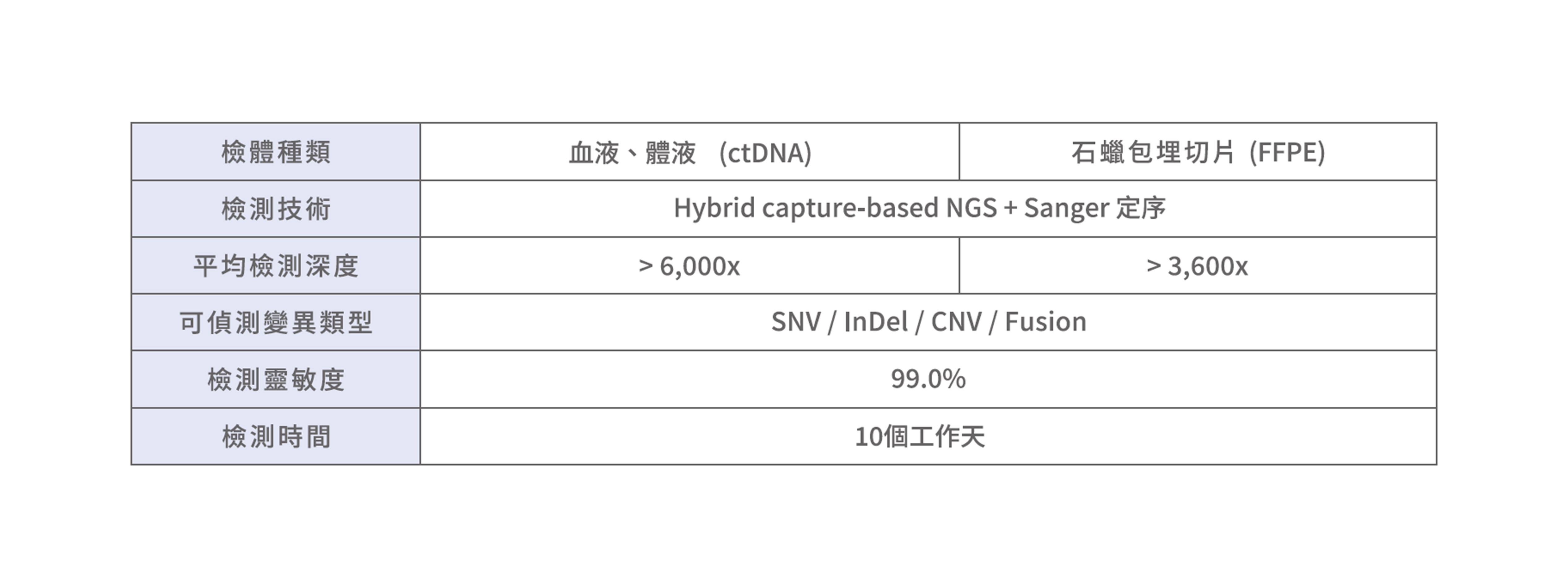
■ 檢測技術可靠: 國際權威驗證
慧智癌監控利用羅氏最新次世代定序CAPP-Seq (Cancer Personalized Profiling by Deep Sequencing)結合專有的綜合數位誤差校正方法(integrated digital error suppression, iDES),因此降低了背景值的干擾,從而提高檢測的特異性,獲得更精確的檢測結果。

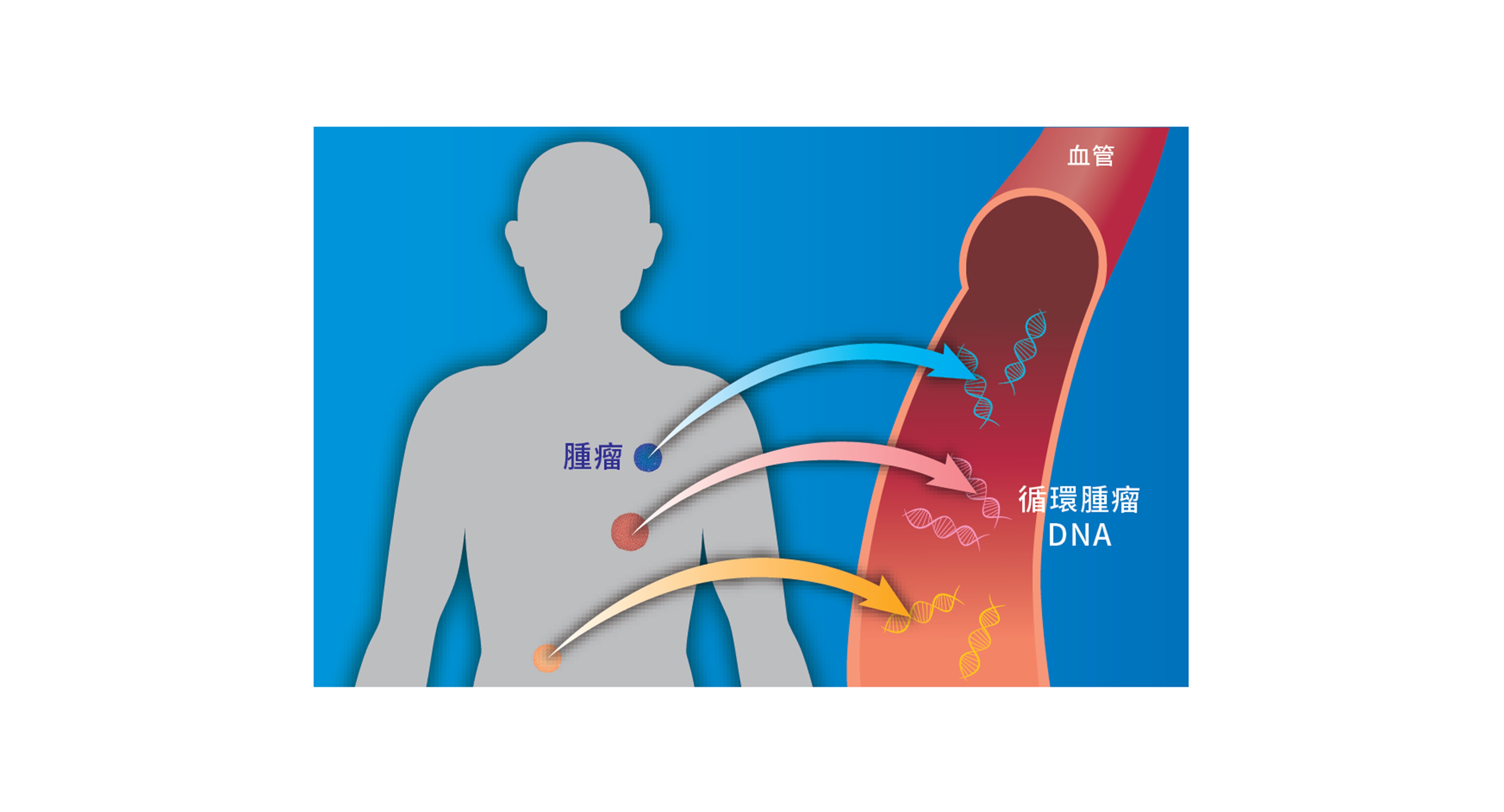
人體中大部分的DNA都存在細胞裡,當細胞死亡後會將DNA釋放到血液中,形成游離DNA或 cfDNA (cell free DNA)。同樣的,當腫瘤細胞死亡後,也會釋放DNA到血液中,這些DNA即為循環腫瘤DNA(circulating tumor DNA, ctDNA)。
而慧智癌監控為因應臨床的需求,提供以下兩種不同的檢體類別:
(1)石蠟切片: 以組織檢體直接進行基因檢測,符合健保申請規範(2)血液、體液: 進行液態切片(Liquid Biopsy)分析,以非侵入的方式取得檢體,並分析。
檢測說明
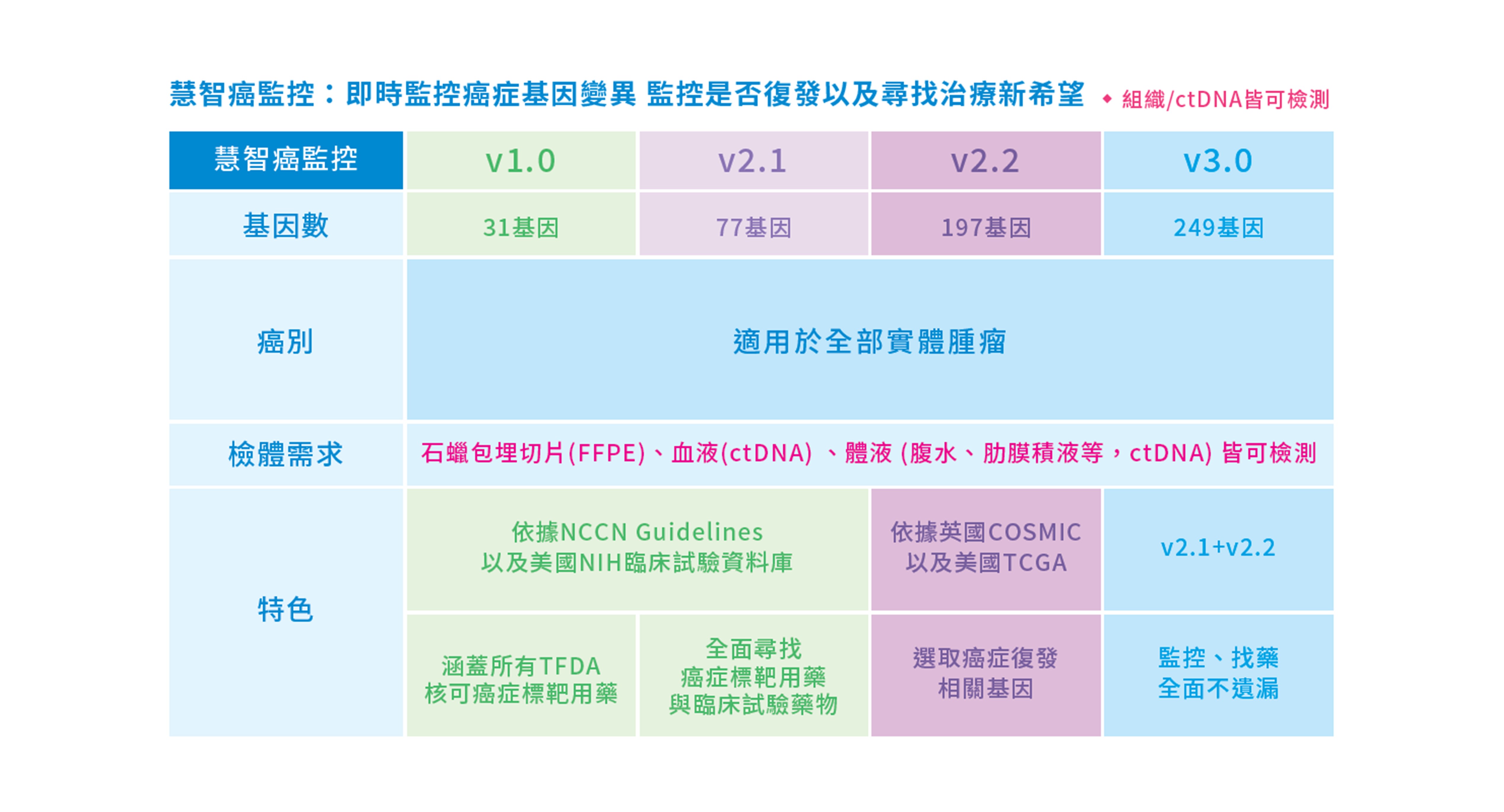
慧智癌監控提供不同基因數量、不同臨床用途的檢測套組,讓病患與醫師討論,選擇最適合的基因檢測,包含:
- 癌監控v1.0: 31個基因,包含食藥署(FDA)核可的所有標靶用藥相關基因。
- 癌監控v2.1: 77個基因,除了已核可的用藥基因外,更包含臨床試驗相關基因。
- 癌監控v2.2: 197個基因,包含25個用藥基因+172個復發相關Minor Gene,可評估預後、療效及監控復發。
- 癌監控v3.0: 249個基因,包含77個用藥基因+172個復發相關Minor Genes,用於尋找標靶治療、臨床試驗、評估療效及監控復發。
適用對象
◆ 所有實體癌症病患
◆ 欲評估標靶治療,或申請健保藥物給付時
◆ 欲找尋標靶治療相關臨床試驗之病患
◆ 欲評估療效、術後輔助治療、抗藥性突變及監控復發之病患。
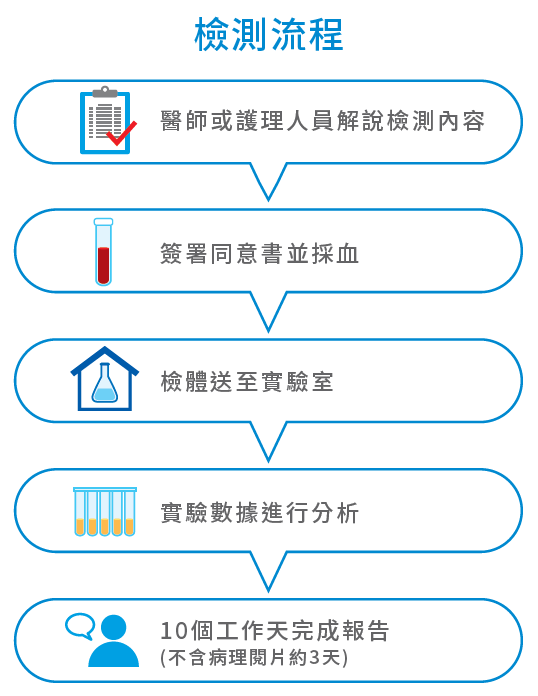
檢測流程
慧智優勢