攝護腺癌基因檢測
找尋標靶治療、評估免疫治療
服務介紹
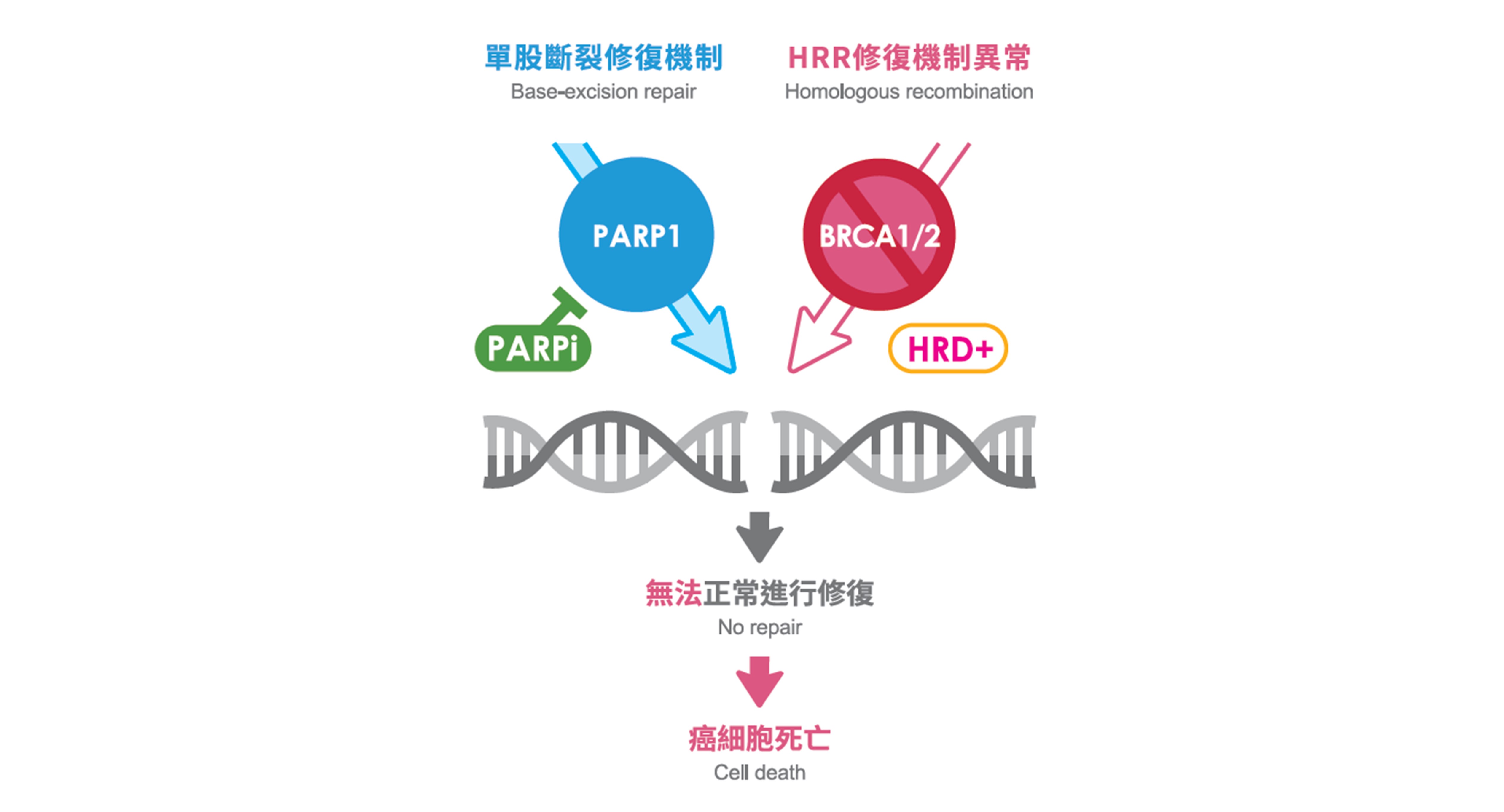
對於轉移性去勢抗性的攝護腺癌病患而言,標靶治療是相當重要的治療手段,相較於傳統的化療,更能降低副作用並達到療效,而在轉移性去勢抗性的攝護腺癌中,約有15%病患帶有BRCA1/2突變,而這些病患若接受PARP抑制劑的標靶治療,就能有較佳的治療效果。而根據美國食藥署(US FDA)及美國國家綜合癌症網絡(NCCN),更歸納出10幾種HRR基因突變適合接受PARP抑制劑的標靶治療,而慧智基因的攝護腺癌基因檢測,除包含BRCA1/2以外,也包含這些HRR基因,可以完整評估PARP抑制劑的標靶治療。
■ 攝護腺癌與免疫治療
除了標靶治療外,免疫治療也成為癌症的治療方式之一,透過活化病患的免疫系統,依靠自身免疫能力清除癌細胞,這樣的治療方式可以適用於不同癌別。而在一個跨國的第二期臨床試驗KEYNOTE-158中,發現帶有高度微衛星不穩定性(MSI-H)的病患接受免疫治療後,能觀察到治療效果,而慧智基因除了針對特定基因進行定序以外,也會針對MSI進行分析,找出適合接受免疫治療的病患。
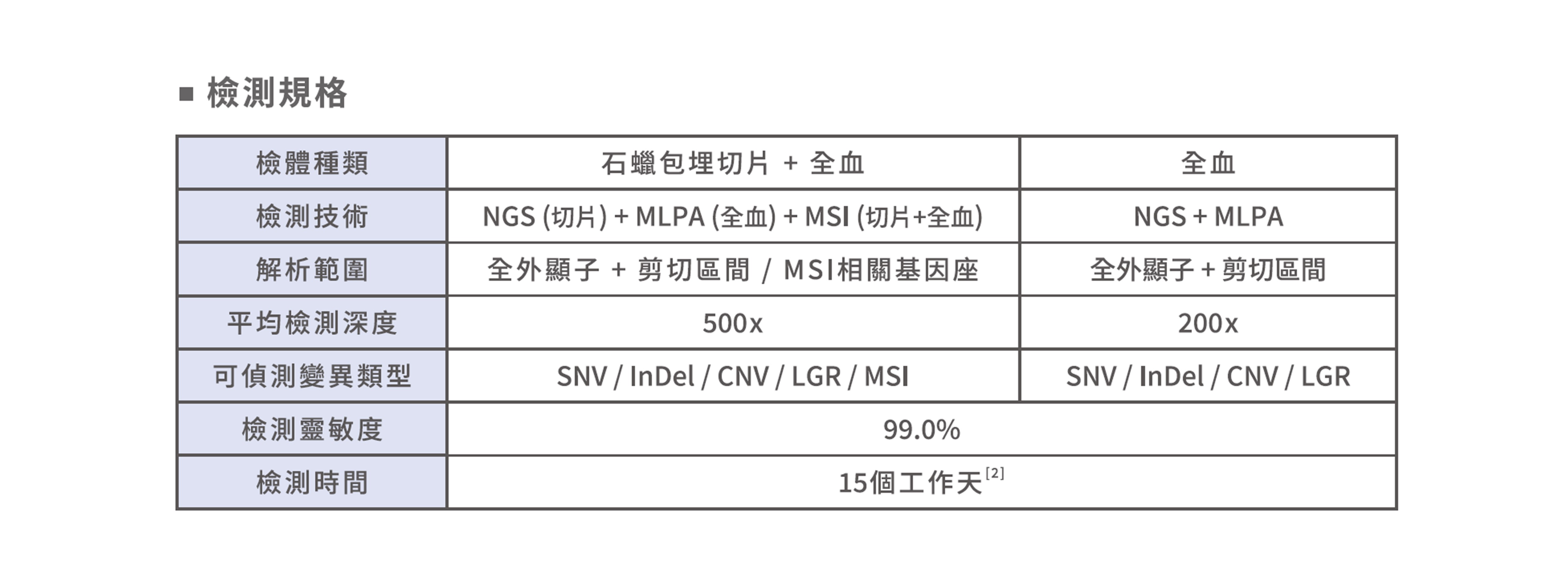
檢測說明
■ 評估攝護腺癌用藥
該檢測包含30個基因,涵蓋PARP抑制劑相關基因、林奇氏症候群(Lynch Syndrome)相關MMR基因以及MSI分析。
適用對象
攝護腺癌病患:
◆ 欲使用PARP抑制劑之攝護腺癌患者。
◆ 欲使用免疫治療pembrolizumab之攝護腺癌患者。
◆ 欲了解自身是否有家族遺傳性Lynch syndrome者。
檢測流程