👑人類乳突病毒篩檢 (HPV)
服務介紹
人類乳突病毒(HPV)是一種DNA病毒,會感染人體的表皮與黏膜組織,透過性接觸傳染,有性行為經驗者皆有機會感染HPV病毒,約8成女性終其一生會感染HPV。
HPV有超過200多種病毒分型,但並不是所有分型都可以導致癌症或疾病,依據致癌能力,HPV可以分為低風險型與高危險型,低風險型較無致癌能力,但特定HPV(如第6、11型)可能導致性傳染病尖銳濕疣(俗稱菜花),而高風險型HPV(特別是第16、18型)容易引起皮膚或黏膜的病變,進而導致癌症的產生,依據感染部位,可能引發的癌症包含子宮頸癌、口咽癌、外陰癌、陰道癌、陰莖癌、肛門癌等。
■ 高風險型HPV與子宮頸癌
九成以上的子宮頸癌是因為HPV感染所導致的,是子宮頸癌最主要的原因,第16、18、31、33、35、39、45、51、52、56、58、59、66、68型HPV更是致癌能力較強的高險型HPV,特別是第16、18型致癌能力最強,約70%的子宮頸癌是因為第16、18型HPV所導致。
■ 什麼是子宮頸癌?該如何預防及早期發現?
子宮頸癌為臺灣女性的第九大癌症,根據國健署的統計,早期子宮頸癌的5年存活率高達9成,但如果癌症末期才發現,則存活率降低至2成,因此如何早期篩檢、早期發現,對婦女健康格外重要。
9成以上的子宮頸癌與人類乳突病毒(HPV)有關,常見預防方式包含以下四種:
建議以上四種預防方式同步進行,接種HPV疫苗並定期進行子宮頸抹片及HPV篩檢,能有效預防、篩檢子宮頸癌。
■ 為什麼要做HPV檢測?
30~40%的子宮頸病變無法透過抹片偵測,HPV篩檢可降低子宮頸抹片的偽陰性
子宮頸抹片經由病理醫師判讀細胞是否病變,但針對早期病變仍可能無法檢出。而HPV檢測採用即時螢光定量聚合酶鏈鎖反應(Real-Time PCR)進行檢測,可增加檢測的準確性並分析HPV的分型,提供更全面的資訊。
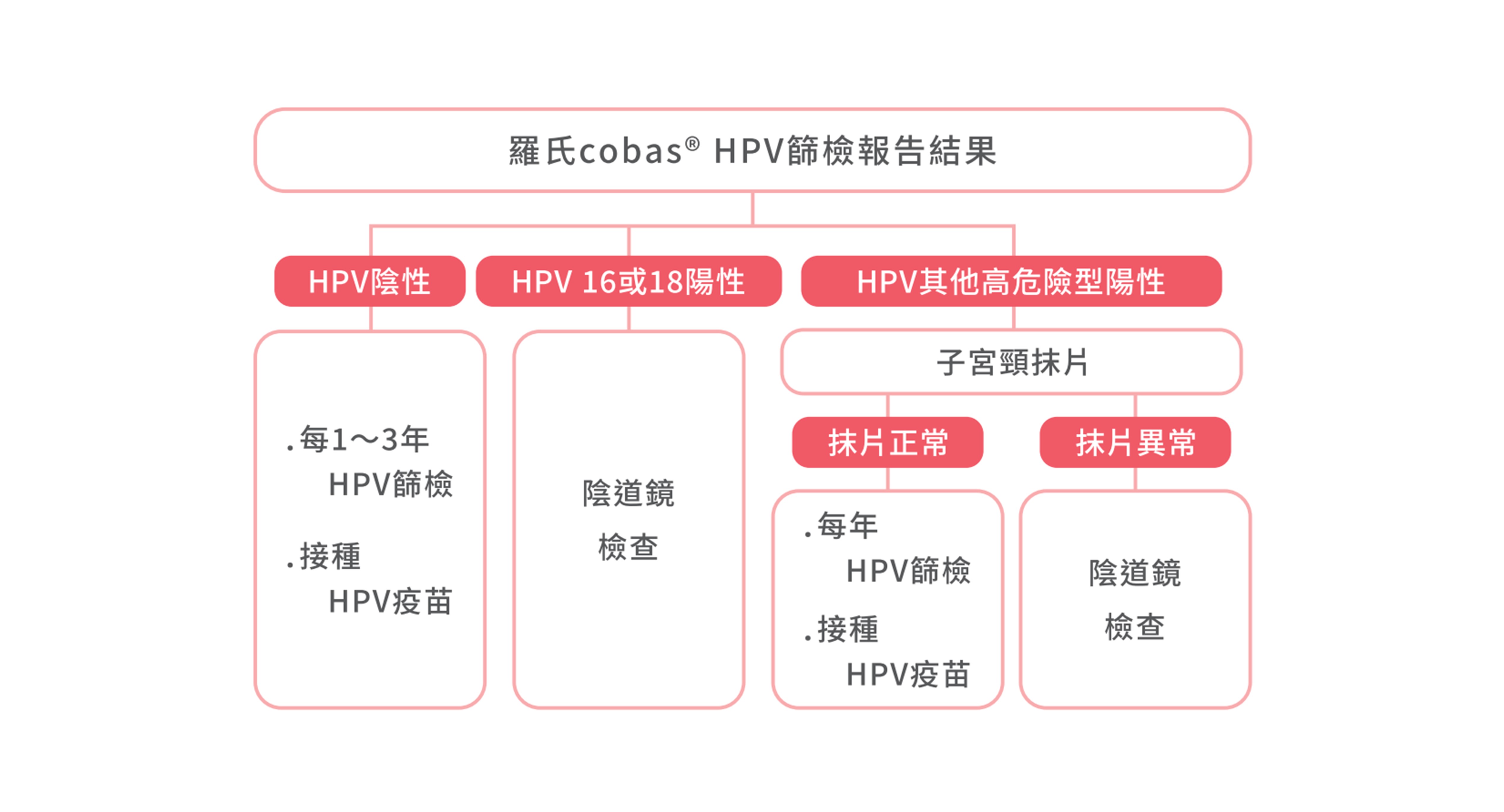
慧智HPV引進羅氏Roche的cobas HPV Test檢測試劑,準確分析病人是否感染HPV第第16、18、31、33、35、39、45、51、52、56、58、59、66、68型型,且有國際臨床研究支持檢測的有效性。完成檢測後,建議至醫療院所接受詳細衛教,評估是否需要安排進一步檢查,例如子宮頸抹片或陰道鏡檢查,並根據分型結果定期進行HPV檢測,追蹤HPV感染情形
檢測說明
慧智使用的羅氏Cobas® HPV篩檢是美國及台灣FDA核准,做為子宮頸癌第一線篩檢工具。一次檢測高危險型HPV,包括16型、18型及其他12種高危險型HPV(31型、33型、35型、39型、45型、51型、52型、56型、58型、59型、66型、68型)。
適用對象
◆ 25歲以上,曾有過性行為之女性
◆ 子宮頸抹片正常,仍想確認是否感染HPV者
◆ 子宮頸抹片異常,欲進行進一步確認者◆ 35、45、65歲婦女欲以健保給付進行HPV檢測者
檢測流程
慧智優勢
