SOFIVA NIPS's Rebranding and Revision
Dear valued customers,
We are delighted to announce the rebranding and revision of our NIPS tests. Please kindly check the details as follows:
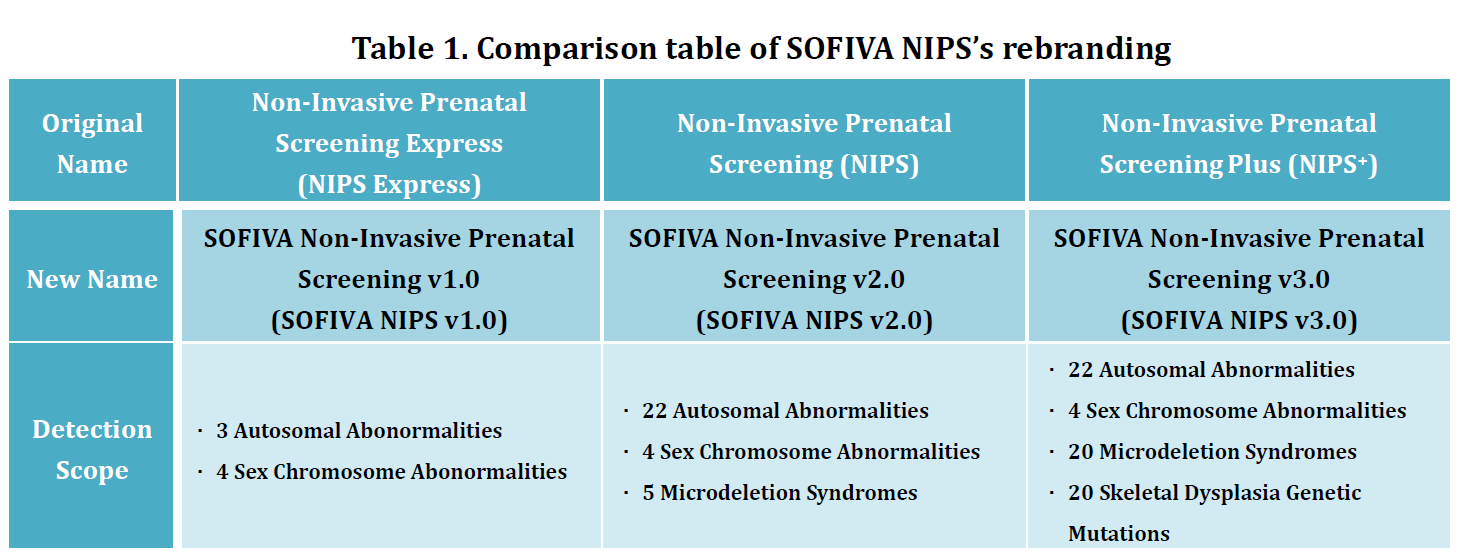
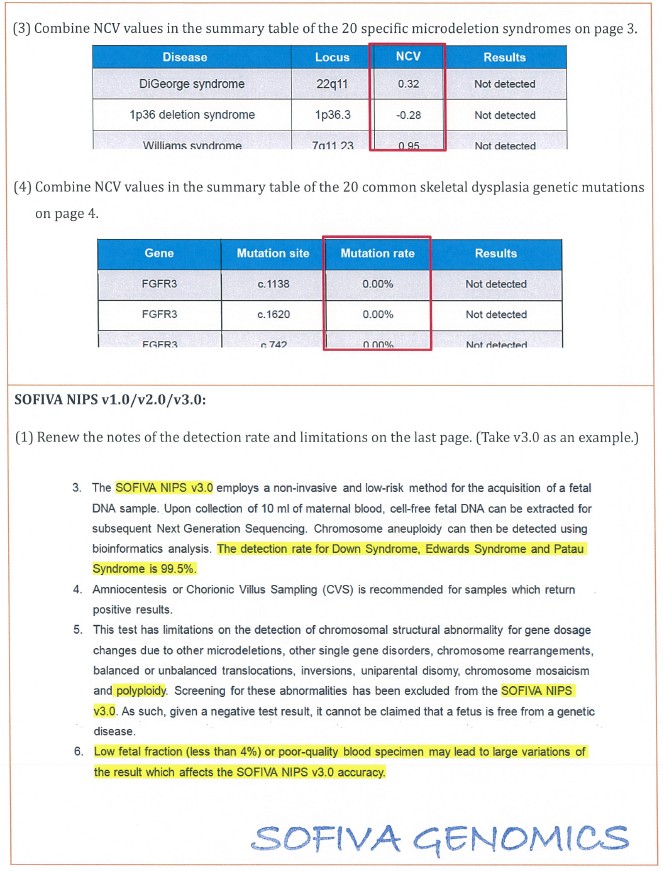
1. The original name of SOFIVA Non-Invasive Prenatal Screening (NIPS) serial products: NIPS Express, NIPS and NIPS+ will be changing to SOFIVA NIPS v1.0, SOFIVA NIPS v2.0 and SOFIVA NIPS v3.0, respectively. (Table 1 ) The scope of detection, price, compensation and after-sales service will remain unchanged.
2. Due to the updates above, the consent form and report of SOFIVA NIPS will be revising.
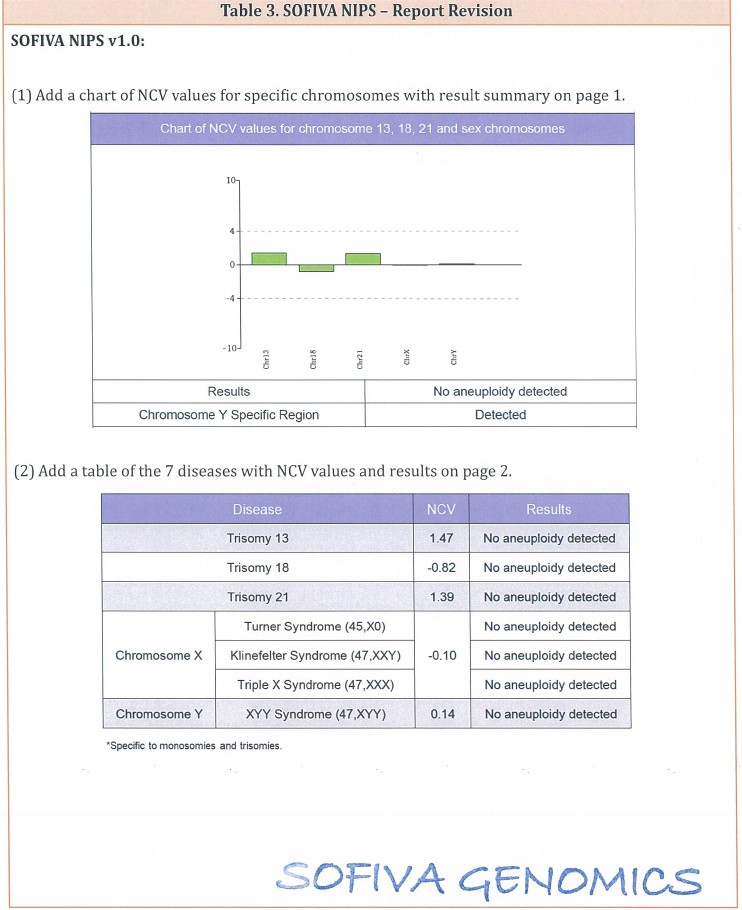
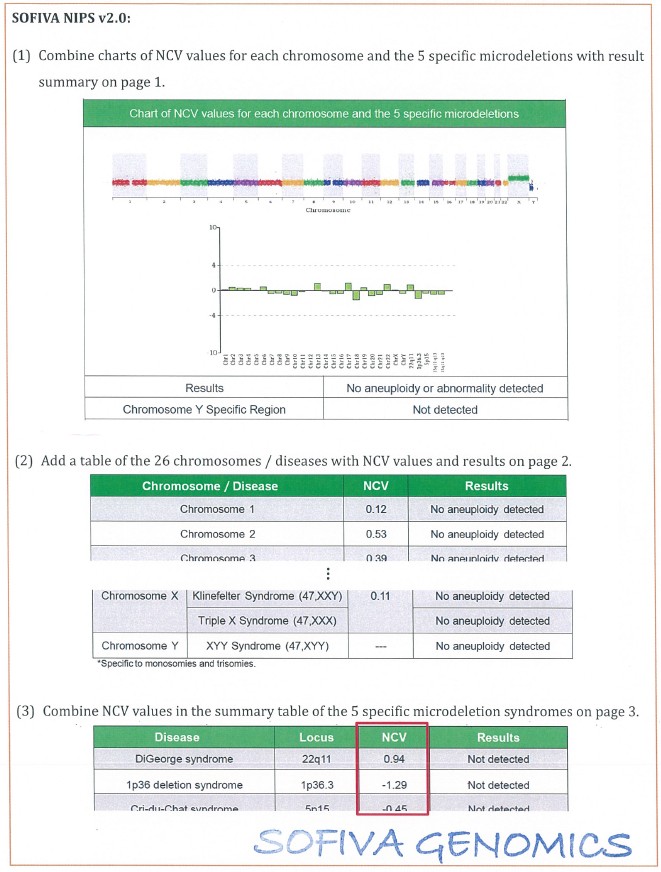
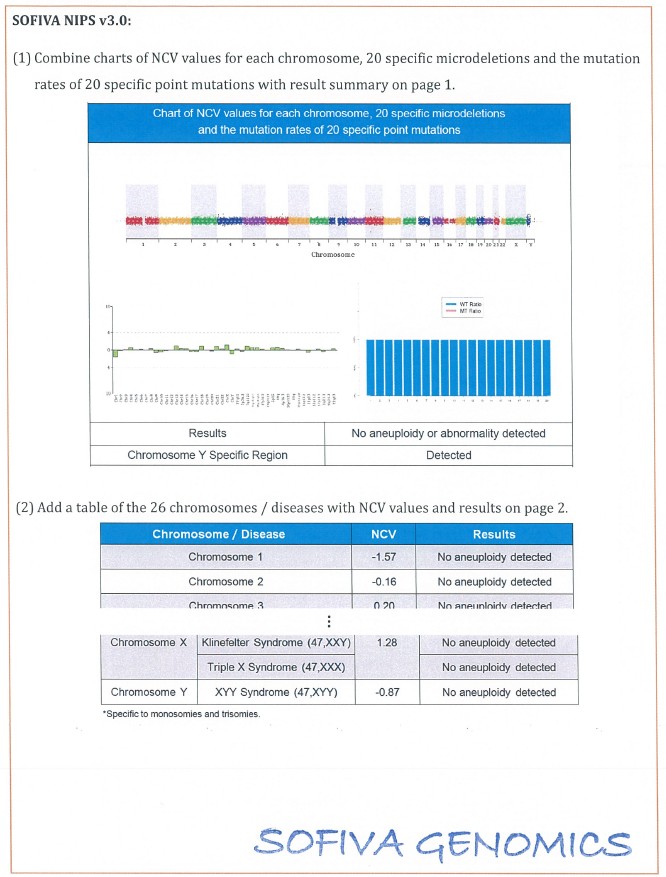
3. The details of revision for the consent form and report are shown in the attachment (Table 2 and Table 3).
4. Patients collected the blood sample from 1st of January 2019 will receive the new version of SOFIVA NIPS report.