Sofiva Genomics Medical Laboratory has obtained TFDA LDTS Registration
Congratulations!
Sofiva Genomics Medical Laboratory has obtained TFDA LDTS Registration on September 28th, 2021.
LDTS (Laboratory Developed Tests and Services) Registration is a quality accreditation set by TFDA (Taiwan Food and Drug Administration) to improve the quality of testing and service in precision medical molecular testing laboratories. The laboratory is checked through multiple procedures such as written review, field visits, and deliberation. Only those who pass the review can publish the registration scope on the TFDA official website.
Obtaining the LDTS registration this time endows SOFIVA GENOMICS higher recognition for its quality of testing and service, also ensures that SOFIVA GENOMICS provides customers with more assurance to highest calibre of testing services. SOFIVA GENOMICS will continue to strive for more diverse quality accreditation in the future.
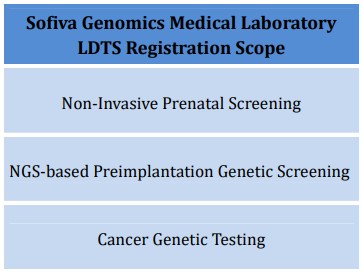
The details of LDTS registration can be inquired through the official website of TFDA